Abstract
INTRODUCTION
The phase 1/2 clinical trials of CD19 CAR T-cell that led to US Food and Drug Administration approval excluded patients with central nervous system (CNS) involvement, due to concerns for CAR T-cell related neurotoxicity. The clinical trial (ChiCTR1800014457) examined efficacy and safety of sequential different B cell antigen-targeted CAR T-cell therapy for pediatric refractory/relapsed (r/r) Burkitt Lymphoma with secondary CNS involvement.
METHODS
Twenty-three patients up to 18 years of age with r/r Burkitt Lymphoma were enrolled between January 17, 2018 and November 8, 2019 including 10 patients with secondary CNS involvement. All enrolled patients received the first mCD19 CAR T-cell infusion. The patients who achieved an ongoing complete response (CR) did not receive further therapy; for patients who retained a partial response (PR) or no response (NR) until mCD19 CAR T cells became undetectable in PB by flow cytometry (FCM), the second hCD22 CAR T-cell infusion was initiated so that it did not prematurely interfere with the antitumor effect of mCD19 CAR T cells, and could prevent disease progression in the setting of continuous mCD19 CAR T-cell contraction; and for patients who had progressive disease (PD) or relapsed disease (RD) after responding to mCD19 CAR T cells, considering that the tumor had already produced immune escape from mCD19 CAR T cells, regardless of whether there were mCD19 CAR T cells detectable in PB by FCM, the second hCD22 CAR T-cell infusion was immediately initiated. After the second hCD22 CAR T-cell infusion, the patients who retained a PR until hCD22 CAR T cells became undetectable in PB by FCM received the third hCD20 CAR T-cell infusion; for patients who suffered relapses after achieving the first CR (CR1) following the first infusion and then attained the second CR (CR2) by the second infusion, the third hCD20 CAR T-cell infusion was given as consolidation therapy after loss of hCD22 CAR T cells detectable in PB by FCM, and the patients who had PD after having obtained a PR immediately received the third hCD20 CAR T-cell infusion even if hCD22 T cells were still detectable in PB by FCM. After the third hCD20 CAR T-cell infusion, the patients who retained a PR until hCD20 CAR T cells became undetectable in PB by FCM were given the fourth hCD19 CAR T-cell infusion.
RESULTS
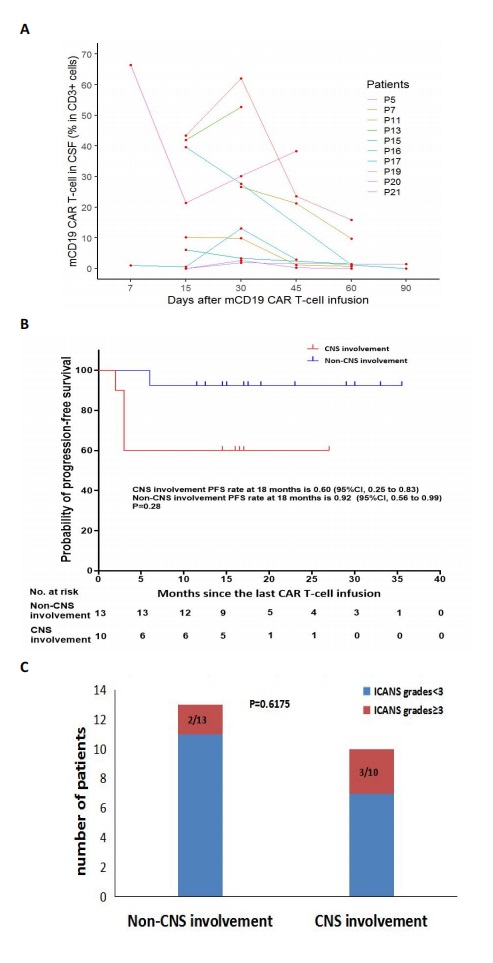
The median time from the last infusion to cutoff date was 17 months (range, 15 to 23). At 18 months after the last infusion, all patients had an estimated PFS rate of 78% (95% CI, 55 to 90) and OS rate of 83% (95% CI, 60 to 93). Through mCD19 CAR T-cell expansion in the cerebrospinal fluids (CSF) of ten patients with CNS involvement, 8 patients achieved a CR, and 2 PR. Among the 8 patients who achieved a CR, 4 patients (P5, P15, P16, and P19) maintained an ongoing CR, whereas 4 patients (P7, P11, P17, and P21 ) developed a RD at m6, m2, m3, and m1.5 after infusion. P17 died of rapid intracerebral mass (ICM) progression, which resulted in elevated intracranial pressure (ICP), a contraindication to further CAR T-cell therapy. Two patients (P7 and P21) with CNS RD, P11 with bone marrow RD, and 2 patients (P13 and P20) with PR received the second hCD22 CAR T cells infusion. Among 5 patients experiencing 2 cycles of CAR T-cell infusion, 4 patients (P7, P13, P20, and P21) had hCD22 CAR T-cell expansion in CSF. P13 achieved an ongoing CR1; P20 and P21 died of ICM progression, who did not proceed with the third hCD20 CAR T-cell infusion due to elevated ICP and loss of CD20 in tumor cells, respectively; P7 and P11 achieved a CR2, who underwent the third hCD20 CAR T cells infusion, and then had hCD20 CAR T cells expansion in CSF. P11 achieved an ongoing CR2; P7 had a CNS RD again at m3 after obtaining a CR2 and finally died of rapid disease progression. Six patients achieved ongoing CR after the last infusion. The estimated 18-month PFS rate of 60% (95% CI, 25 to 83) in the patients with CNS involvement was not significantly lower than 92% (95% CI, 56 to 99) in the patients without CNS involvement (p=0.28 ). Grade 3 neurotoxicity occurred in 30% (3/10) of patients with CNS involvement. All adverse events were reversible. Statistically significant difference was not observed in the severity of neurotoxicity between patients with and without CNS involvement (p= 0.62).
CONCLUSION
Sequential CAR T-cell therapy may result in a durable response and is safe in pediatric r/r Burkitt lymphoma. Patients with secondary CNS involvement may benefit from sequential CAR T-cell therapy.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal